
Our paper “In situ analysis of type III secretion chaperone proteins indicates a cytosolic handover of virulence effectors” has been voted "Best Paper of 2025" in FEMS Microbes. Congratulation to the first author, Keith, and thanks to all collaborators on this project!
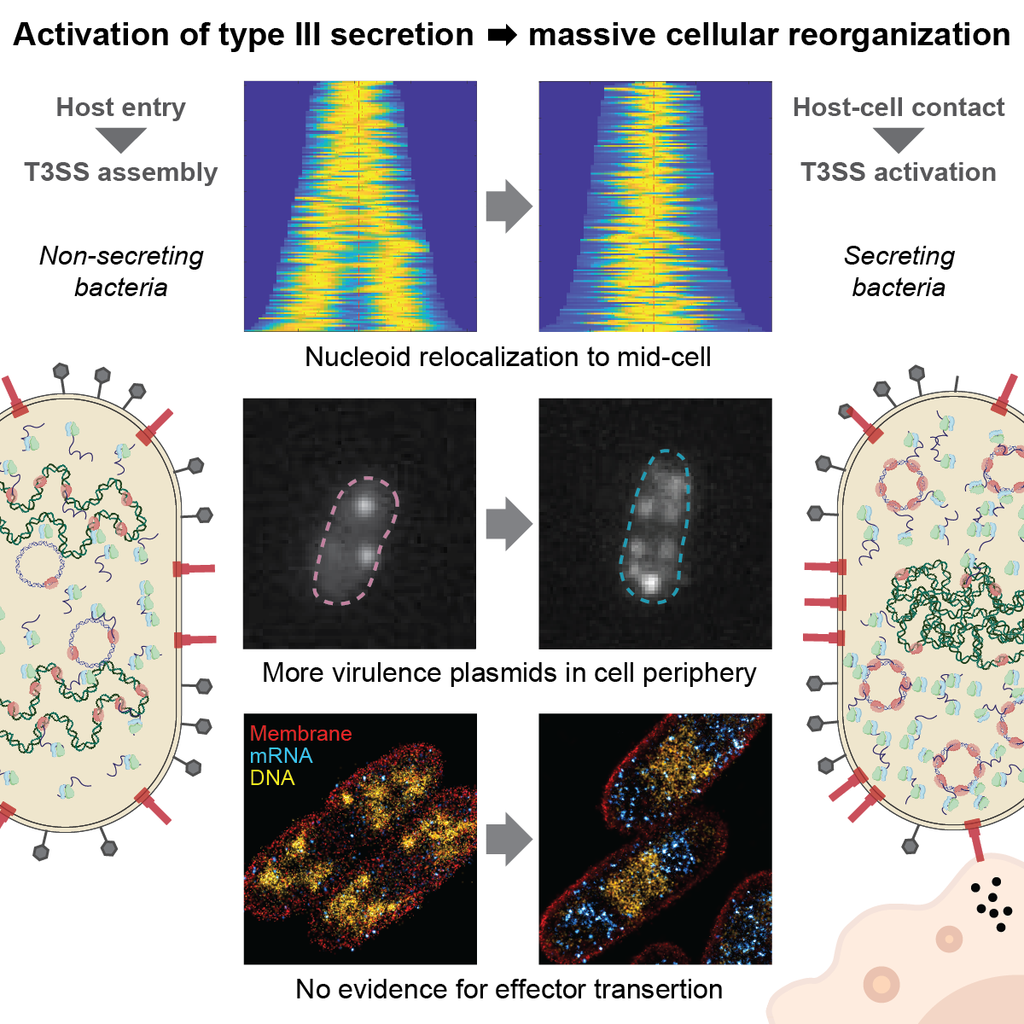
Secreting bacteria undergo drastic changes.
We found that in Yersinia, T3SS activation triggers rapid, large-scale reorganisation of chromosomal and plasmid DNA. This links secretion to growth inhibition-revealing a new connection between virulence and bacterial cell biology. Our paper "Activation of the Yersinia type III secretion system induces large-scale spatial reorganization of chromosomal and virulence plasmid DNA" has just been published in Cell Reports.
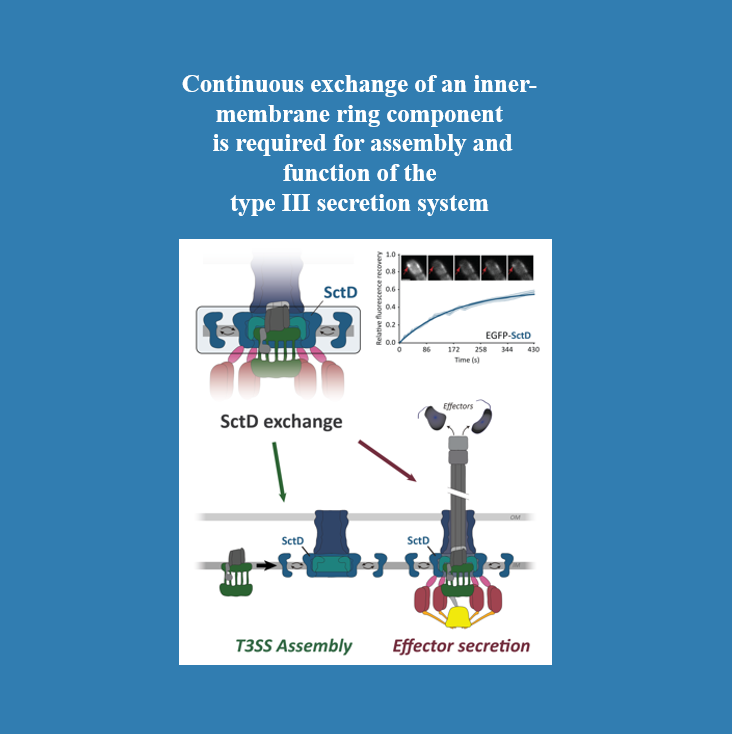
What if even core parts of bacterial nanomachines were not as static as we thought?
Molecular nanomachines such as the Type III Secretion System (T3SS) were long believed to function as static mechanical engines. While some soluble T3SS components were already known to be dynamic, we now provide surprising evidence that the core structural T3SS component SctD continuously exchanges subunits under native conditions. Engineering a crosslinkable protein variant revealed that SctD dynamics is required for proper T3SS assembly and function.
Curious to learn more about the T3SS and how our discovery challenges long-held assumptions? Read the full story in our new Nature Communications paper: "Continuous exchange of an inner-membrane ring component is required for assembly and function of the type III secretion system"
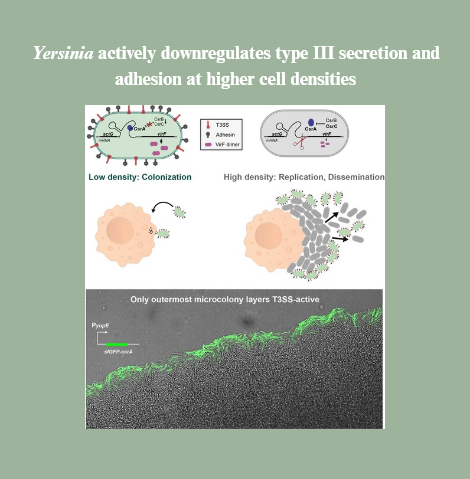
T3SS activity comes at a cost — bacteria that use the system pay with reduced growth. We found out how Yersinia balance costs and benefits: at higher densities, the bacteria actively suppress T3SS activity and adhesion, switching from a colonization to replication and dissemination phase.
Our paper "Yersinia actively downregulates type III secretion and adhesion at higher cell densities" has just been published in PLOS Pathogens! 👏
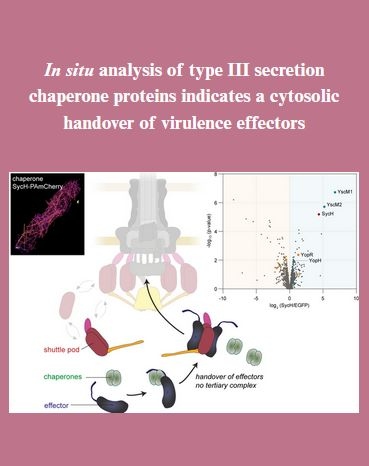
How do type III secretion effectors get recruited to the injectisome? 🤔
Last year, we uncovered a shuttle system that specifically captures them in the cytosol and delivers them to the export gate at the injectisome.
But what about the chaperones that usually bind the effectors? Katherine Pintor in our lab investigated them with live cell microscopy, proximity labeling and functional assays. We found that the chaperone of the Yersinia effector YopH does not take part in the journey and just hands over its cargo to the shuttles which is a further step towards understanding how the T3SS works on the molecular level!
🧬Our paper "In situ analysis of type III secretion chaperone proteins indicates a cytosolic handover of virulence effectors" has now been published in FEMS Microbes.
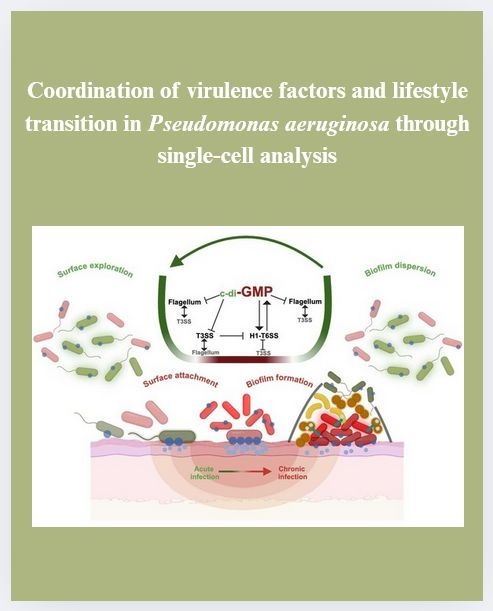
Our first accepted manuscript at our new lab in Karlsruhe has just been published in Communications Biology!
In this study, we investigated how Pseudomonas aeruginosa orchestrates different virulence systems, the T3SS, H1-T6SS, and the flagellum. Using single-cell microscopy, we uncovered that distinct bacterial subpopulations coordinate these molecular machineries, all tuned by the usual suspect: cyclic di-GMP.
Read for yourself: "Coordination of virulence factors and lifestyle transition in Pseudomonas aeruginosa through single-cell analysis".
This is the first of three recently accepted manuscripts & stay tuned for the next two!👀

This Saturday, we had the pleasure of participating in Campus Day 2025. Visitors with an interest in science were treated to a captivating talk by Prof. Diepold, who shared fascinating insights into the clever strategies bacteria use to infect humans. His presentation provided an excellent overview of bacterial secretion systems and offered a glimpse into the work being done in our lab.
In addition to the talk, guests had the opportunity to engage with Kira and Corentin, who presented posters showcasing their research projects. Their discussions sparked many interesting conversations and further highlighted the exciting work happening in our team.
Overall, it was a wonderful day filled with inspiring science and great interactions. We’re grateful to everyone who stopped by!

We’re excited to welcome our first students to the institute! Natalie is completing her F3 internship, while Kaize, Leon, and Madelaine are doing their BA08 internship before their bachelor's thesis. It’s amazing to see our institute grow – and even better to guide and support new people along the way. Here’s to a great journey together!
